Assuming constant pressure, ΔH is a measure of the change of heat energy during a reaction. Since ΔH is negative, energy was released during the reaction therefore this reaction is exothermic. Suppose 20 g of H2O2 react. Will heat be released or absorbed?
16 Science Experiments to Teach About Electricity | Science Buddies Blog
Apr 12, 2023Expand/collapse global location

Source Image: tes.com
Download Image
A chemist measures the energy change during the following reaction: C3H8 (g) + 5O2 (g) –> 3CO2 (g) + 4H2O (l) DeltaH = -2220 kJ Use the information to answer the following questions. — Is the reaction endothermic or exothermic? — Suppose 49.8 g of C3H8 react. Will heat be released or absorbed?

Source Image: m.youtube.com
Download Image
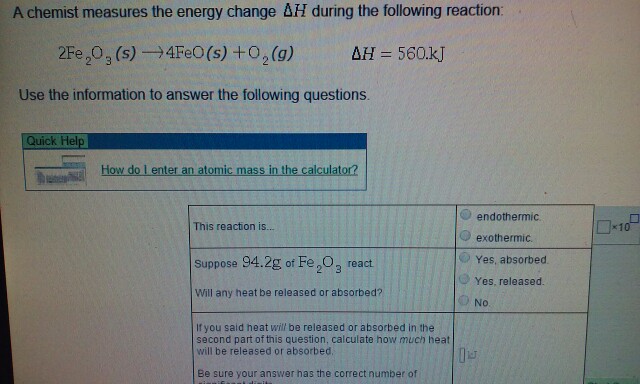
Pin em Thermochemistry A chemist measures the energy change ∆H during the following reaction \ce 2Fe2O3 (s)\rightarrow 4FeO (s)+O2 (g) 2FeX 2OX 3(s) → 4FeO(s)X +OX 2(g) and it is equal to ∆H=560\ \pu kJ ∆H = 560 kJ a) This reaction is: Endothermic or exothermic. b) Suppose 94.2\ \pu g 94.2 g of \ce Fe2O3 FeX 2OX 3 reacts. Will any heat be released or absorbed?
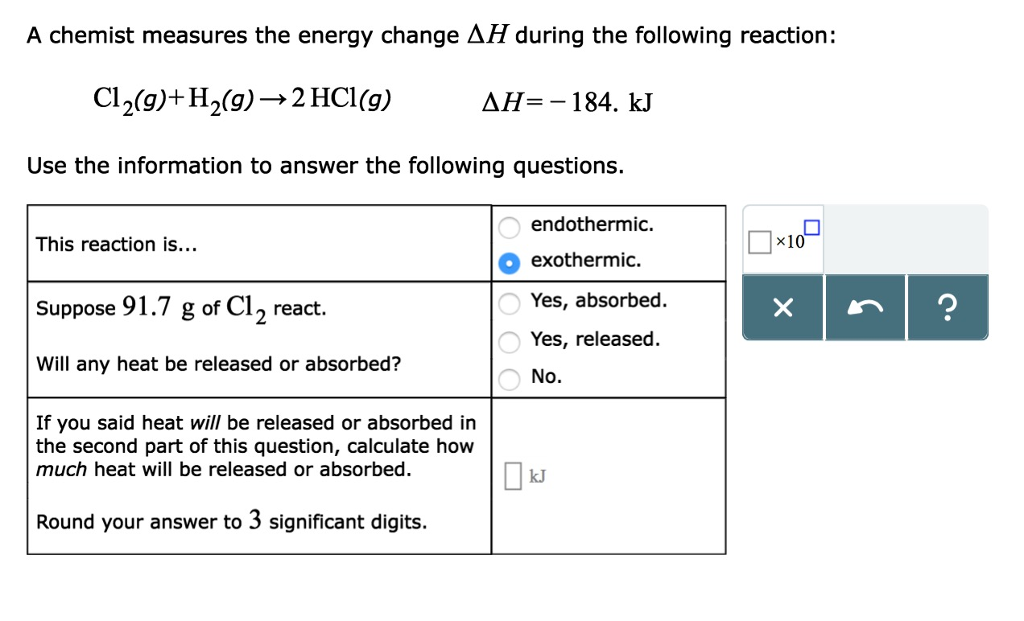
Source Image: chegg.com
Download Image
A Chemist Measures The Energy Change During The Following Reaction:
A chemist measures the energy change ∆H during the following reaction \ce 2Fe2O3 (s)\rightarrow 4FeO (s)+O2 (g) 2FeX 2OX 3(s) → 4FeO(s)X +OX 2(g) and it is equal to ∆H=560\ \pu kJ ∆H = 560 kJ a) This reaction is: Endothermic or exothermic. b) Suppose 94.2\ \pu g 94.2 g of \ce Fe2O3 FeX 2OX 3 reacts. Will any heat be released or absorbed? A chemist measures the energy change Δ H during the following reacti C 3 H 8 ( g ) + 5 O 2 ( g ) → 3 C O 2 ( g ) + 4 H 2 O ( I ) , Δ H = – 2 2 2 0 . k J Use the information to answer the following questions.
Solved A chemist measures the energy change ΔH during the | Chegg.com
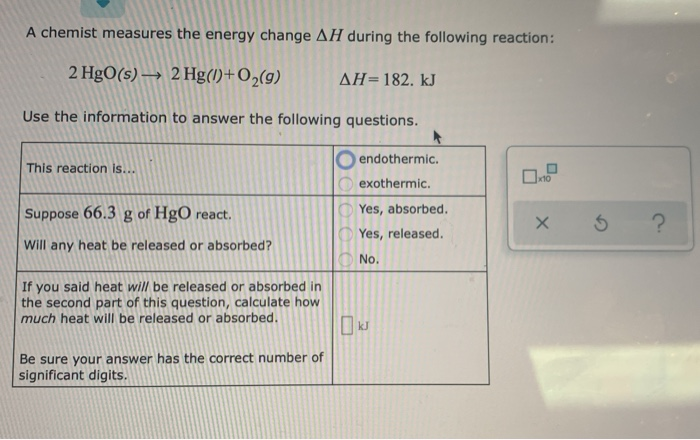
Question: A chemist measures the energy change ?H during the following reaction: 2HgO (s) ?2Hg (l) +O2 (g) =?H182.kJ Use the information to answer the following questions. This reaction is… endothermic. exothermic. Suppose 72.8g of HgO react. Will any heat be released or absorbed? Yes, absorbed. Yes, released. No. One-Pot Wonder: The New Nanosheet Method Catalyzing a Green Energy Revolution
Source Image: scitechdaily.com
Download Image
Solved A chemist measures the energy change Delta H during | Chegg.com Question: A chemist measures the energy change ?H during the following reaction: 2HgO (s) ?2Hg (l) +O2 (g) =?H182.kJ Use the information to answer the following questions. This reaction is… endothermic. exothermic. Suppose 72.8g of HgO react. Will any heat be released or absorbed? Yes, absorbed. Yes, released. No.
Source Image: chegg.com
Download Image
16 Science Experiments to Teach About Electricity | Science Buddies Blog Assuming constant pressure, ΔH is a measure of the change of heat energy during a reaction. Since ΔH is negative, energy was released during the reaction therefore this reaction is exothermic. Suppose 20 g of H2O2 react. Will heat be released or absorbed?

Source Image: sciencebuddies.org
Download Image
Pin em Thermochemistry A chemist measures the energy change during the following reaction: C3H8 (g) + 5O2 (g) –> 3CO2 (g) + 4H2O (l) DeltaH = -2220 kJ Use the information to answer the following questions. — Is the reaction endothermic or exothermic? — Suppose 49.8 g of C3H8 react. Will heat be released or absorbed?

Source Image: pinterest.com
Download Image
Premium Vector | Changes in gibbs free energy depicted in a reaction diagram of a thermodynamically favorable rxn Find stepby-step Chemistry solutions and your answer to the following textbook question: A chemist measures the energy change $\Delta H$ during the following reaction: $\ce2 HgO(s) -> 2Hg(l) + O2(g) \qquad \Delta H =182 kJ$ Suppose 72.6 g of HgO reacts. How much heat will be released or absorbed?.

Source Image: freepik.com
Download Image
Solved A chemist measures the energy change AH during the | Chegg.com A chemist measures the energy change ∆H during the following reaction \ce 2Fe2O3 (s)\rightarrow 4FeO (s)+O2 (g) 2FeX 2OX 3(s) → 4FeO(s)X +OX 2(g) and it is equal to ∆H=560\ \pu kJ ∆H = 560 kJ a) This reaction is: Endothermic or exothermic. b) Suppose 94.2\ \pu g 94.2 g of \ce Fe2O3 FeX 2OX 3 reacts. Will any heat be released or absorbed?
Source Image: chegg.com
Download Image
Edexcel IGCSE Chemistry Worksheet 26 – Energetics | Teaching Resources A chemist measures the energy change Δ H during the following reacti C 3 H 8 ( g ) + 5 O 2 ( g ) → 3 C O 2 ( g ) + 4 H 2 O ( I ) , Δ H = – 2 2 2 0 . k J Use the information to answer the following questions.

Source Image: tes.com
Download Image
Solved A chemist measures the energy change Delta H during | Chegg.com
Edexcel IGCSE Chemistry Worksheet 26 – Energetics | Teaching Resources Apr 12, 2023Expand/collapse global location
Pin em Thermochemistry Solved A chemist measures the energy change AH during the | Chegg.com Find stepby-step Chemistry solutions and your answer to the following textbook question: A chemist measures the energy change $\Delta H$ during the following reaction: $\ce2 HgO(s) -> 2Hg(l) + O2(g) \qquad \Delta H =182 kJ$ Suppose 72.6 g of HgO reacts. How much heat will be released or absorbed?.