Classify these diatomic molecules as diamagnetic or paramagnetic: O2, F2, B2, C2, N2 Solution Verified Create a free account to view solutions Continue with Google Recommended textbook solutions Chemistry: The Molecular Nature of Matter and Change 7th Edition • ISBN: 9780073511177 Patricia Amateis, Silberberg 6,032 solutions
Paramagnetic vs Diamagnetic – Paired vs Unpaired Electrons – Electron Configuration – YouTube
Oct 19, 2022The same principle applies to compounds. A compound that has unpaired electrons is paramagnetic, while one with no unpaired electrons is diamagnetic. Ammonia (NH 3) is an example of a diamagnetic compound. The coordination complex [Fe (edta) 3 )] 2- is an example of a paramagnetic compound. Paramagnetic.
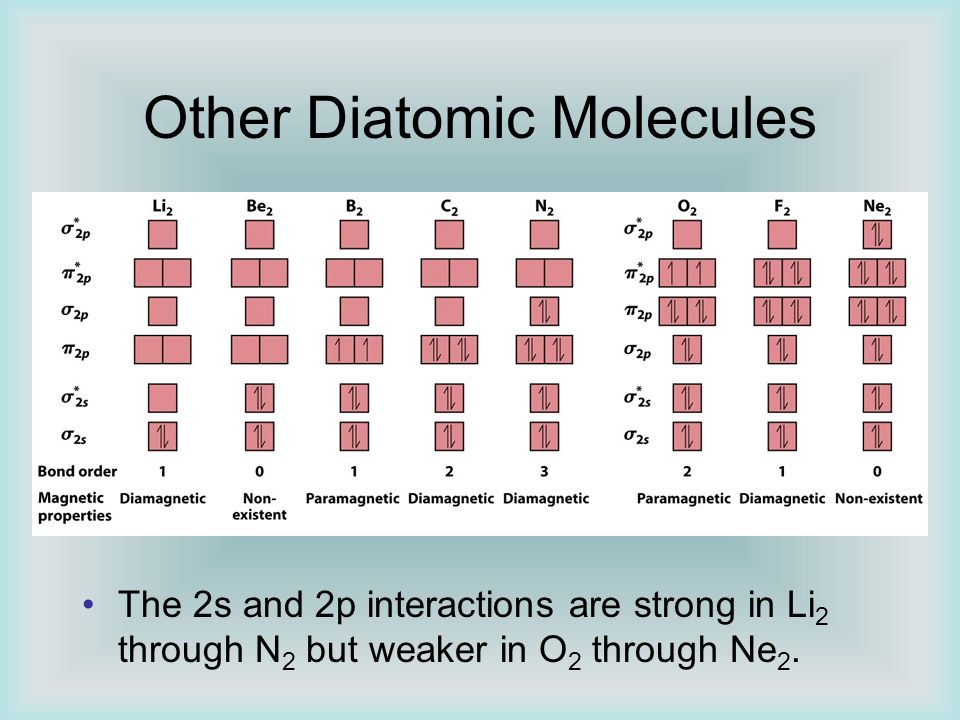
Source Image: slideplayer.com
Download Image
One explanation I read is that “Hemoglobin without bound oxygen molecules, deoxyhemoglobin, is paramagnetic because of the high spin state (S = 2) of the heme iron. In contrast, oxygen-bound hemoglobin, oxyhemoglobin, has low spin (S = 0) and is diamagnetic (Pauling &Coryl 1936). ” I know the iron in heme is Fe2+.

Source Image: m.youtube.com
Download Image
Paramagnetic, Diamagnetic And Ferromagnetic Substances – Magnets By HSMAG . Assuming that the two beams reflect from the same family of reflecting planes, find (a) the interplanar spacing and (b) the wavelength A. college algebra For the following exercises, express the equation for the hyperbola as two functions, with y as a function of x. Express as simply as possible.
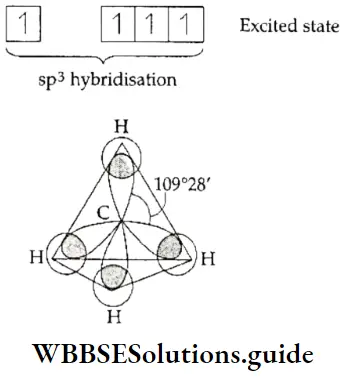
Source Image: wbbsesolutions.guide
Download Image
Classify These Diatomic Molecules As Diamagnetic Or Paramagnetic
. Assuming that the two beams reflect from the same family of reflecting planes, find (a) the interplanar spacing and (b) the wavelength A. college algebra For the following exercises, express the equation for the hyperbola as two functions, with y as a function of x. Express as simply as possible. Science Physics Physics questions and answers Classify these diatomic molecules as diamagnetic or paramagnetic.ParamaoneticAnswer BankN2B2C2F2 This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer
Class 11 Chemistry Archives – Page 3 of 5 – WBBSE Solutions
Chem 321: Physical Chemistry I Chemistry Pages About Elements, their Naming, and Properties | SKM Classes Bangalore
Source Image: zookeepersblog.wordpress.com
Download Image
Bond-order and Magnetic Behavior of Diatomic Species without Molecular Orbital Theory Chem 321: Physical Chemistry I

Source Image: pubs.sciepub.com
Download Image
Paramagnetic vs Diamagnetic – Paired vs Unpaired Electrons – Electron Configuration – YouTube Classify these diatomic molecules as diamagnetic or paramagnetic: O2, F2, B2, C2, N2 Solution Verified Create a free account to view solutions Continue with Google Recommended textbook solutions Chemistry: The Molecular Nature of Matter and Change 7th Edition • ISBN: 9780073511177 Patricia Amateis, Silberberg 6,032 solutions
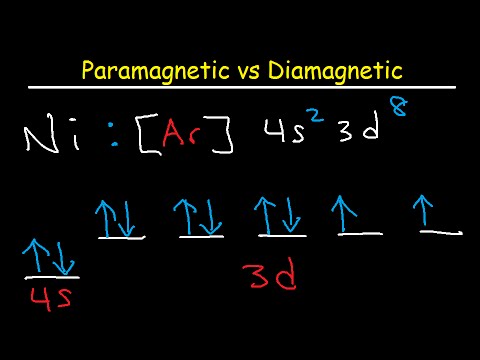
Source Image: youtube.com
Download Image
Paramagnetic, Diamagnetic And Ferromagnetic Substances – Magnets By HSMAG One explanation I read is that “Hemoglobin without bound oxygen molecules, deoxyhemoglobin, is paramagnetic because of the high spin state (S = 2) of the heme iron. In contrast, oxygen-bound hemoglobin, oxyhemoglobin, has low spin (S = 0) and is diamagnetic (Pauling &Coryl 1936). ” I know the iron in heme is Fe2+.

Source Image: hsmagnets.com
Download Image
Recent studies on the magnetic properties of paramagnetic metals linked by diamagnetic second metals – ScienceDirect Jul 12, 2023A magnetic moment is a vector quantity, with a magnitude and a direction. An electron has an electron magnetic dipole moment, generated by the electron’s intrinsic spin property, making it an electric charge in motion. There are many different magnetic forms: including paramagnetism, and diamagnetism, ferromagnetism, and anti-ferromagnetism.

Source Image: sciencedirect.com
Download Image
Class 11 Chemistry Archives – Page 3 of 5 – WBBSE Solutions . Assuming that the two beams reflect from the same family of reflecting planes, find (a) the interplanar spacing and (b) the wavelength A. college algebra For the following exercises, express the equation for the hyperbola as two functions, with y as a function of x. Express as simply as possible.
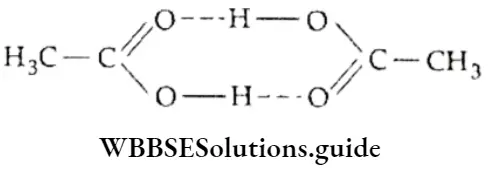
Source Image: wbbsesolutions.guide
Download Image
Which of the following diatomic species are paramagnetic and which are diamagnetic? A blank molecular – brainly.com Science Physics Physics questions and answers Classify these diatomic molecules as diamagnetic or paramagnetic.ParamaoneticAnswer BankN2B2C2F2 This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer

Source Image: brainly.com
Download Image
Bond-order and Magnetic Behavior of Diatomic Species without Molecular Orbital Theory
Which of the following diatomic species are paramagnetic and which are diamagnetic? A blank molecular – brainly.com Oct 19, 2022The same principle applies to compounds. A compound that has unpaired electrons is paramagnetic, while one with no unpaired electrons is diamagnetic. Ammonia (NH 3) is an example of a diamagnetic compound. The coordination complex [Fe (edta) 3 )] 2- is an example of a paramagnetic compound. Paramagnetic.
Paramagnetic, Diamagnetic And Ferromagnetic Substances – Magnets By HSMAG Class 11 Chemistry Archives – Page 3 of 5 – WBBSE Solutions Jul 12, 2023A magnetic moment is a vector quantity, with a magnitude and a direction. An electron has an electron magnetic dipole moment, generated by the electron’s intrinsic spin property, making it an electric charge in motion. There are many different magnetic forms: including paramagnetism, and diamagnetism, ferromagnetism, and anti-ferromagnetism.