The bond-making between the nucleophile and the electrophilic carbon occurs at the same time as the bond-breaking between the electophilic carbon and the halogen. In order of decreasing importance, the factors impacting S N 2 reaction pathways are. 1) structure of the alkyl halide. 2) strength of the nucleophile. 3) stability of the leaving group.
Solved Which SN2 reaction in each pair is faster? a. CH CH | Chegg.com
Chemistry Chemistry questions and answers Which Sn2 reaction is each pair is faster? А. Оосно Br О. + Br Br Qocня. Br В. ci с сн,соо + Өон СІ ОН + This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Question: Which Sn2 reaction is each pair is faster? А.

Source Image: pinterest.com
Download Image
Solution Verified by Toppr Correct option is A) Solve any question of Haloalkanes and Haloarenes with:- Patterns of problems > Was this answer helpful? 0 0 Similar questions Which of the following compounds would react faster with NaCN in an S N 2 reaction? Medium View solution >

Source Image: sciencedirect.com
Download Image
Which would undergo SN2 reaction faster in the above pair and Why? Lesson 2: SN2. Sn2 mechanism: kinetics and substrate. Factors affecting SN2 reactions: substrate effect. Sn2 mechanism: stereospecificity. Effect of substrate of SN2 reactions. Factors affecting SN2 reactions: leaving group- Part 1. Factors affecting SN2 reactions: leaving group-Part 2. Factors affecting SN2 reactions: Leaving group- Part 3.

Source Image: m.youtube.com
Download Image
Which Sn2 Reaction In The Following Pair Is Faster
Lesson 2: SN2. Sn2 mechanism: kinetics and substrate. Factors affecting SN2 reactions: substrate effect. Sn2 mechanism: stereospecificity. Effect of substrate of SN2 reactions. Factors affecting SN2 reactions: leaving group- Part 1. Factors affecting SN2 reactions: leaving group-Part 2. Factors affecting SN2 reactions: Leaving group- Part 3. Which compound in each of the following pairs will react faster in S N 2 reaction with ¯ O H? C H 3 B r o r C H 3 I (C H 3) 3 C C l o r C H 3 C l. Which compound in each of the following pairs will react faster in S N 2 reaction with ¯ O H?
Which Reaction Faster? SN1 or SN2? – YouTube
7: Nucleophilic Substitution Reactions Which compound in this pair undergoes a faster SN2 reaction? | Homework.Study.com

Source Image: homework.study.com
Download Image
Predict the compound in each pair that will undergo the SN2 react… | Channels for Pearson+ 7: Nucleophilic Substitution Reactions

Source Image: pearson.com
Download Image
Solved Which SN2 reaction in each pair is faster? a. CH CH | Chegg.com The bond-making between the nucleophile and the electrophilic carbon occurs at the same time as the bond-breaking between the electophilic carbon and the halogen. In order of decreasing importance, the factors impacting S N 2 reaction pathways are. 1) structure of the alkyl halide. 2) strength of the nucleophile. 3) stability of the leaving group.
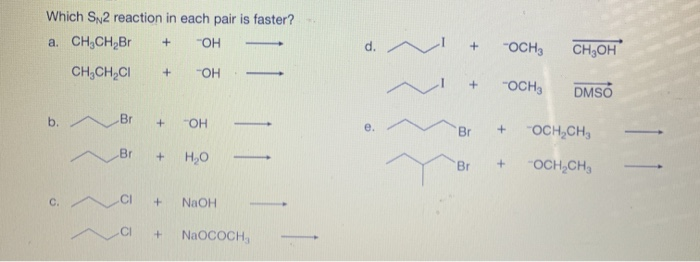
Source Image: chegg.com
Download Image
Which would undergo SN2 reaction faster in the above pair and Why? Solution Verified by Toppr Correct option is A) Solve any question of Haloalkanes and Haloarenes with:- Patterns of problems > Was this answer helpful? 0 0 Similar questions Which of the following compounds would react faster with NaCN in an S N 2 reaction? Medium View solution >
Source Image: toppr.com
Download Image
Comparing The SN1 vs Sn2 Reactions – Master Organic Chemistry Jan 11, 20241) The reaction below follows the S N 2 mechanism. a) Write the rate law for this reaction. b) Determine the value of the rate coefficient, k, if the initial concentrations are 0.01 M CH 3 Cl, 0.01 M NaOH, and the initial reaction rate is 6 x 10 -10 M/s. c) Calculate the initial reaction rate if the initial reactant concentrations are changed
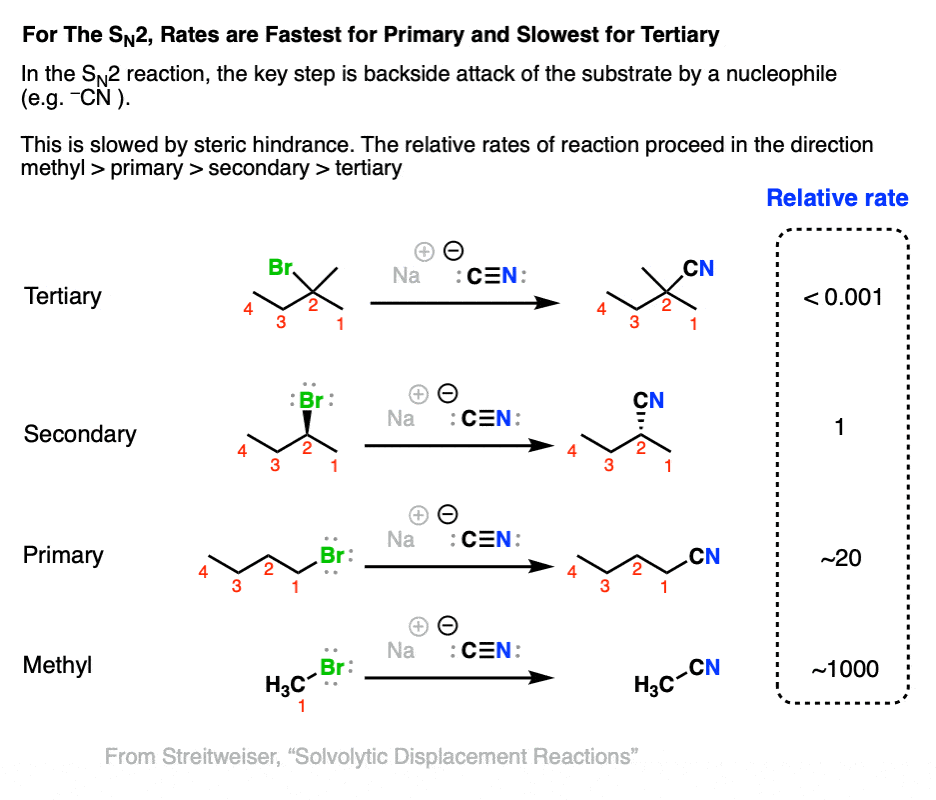
Source Image: masterorganicchemistry.com
Download Image
Chapter 8: Nucleophilic Substitution – ppt download Lesson 2: SN2. Sn2 mechanism: kinetics and substrate. Factors affecting SN2 reactions: substrate effect. Sn2 mechanism: stereospecificity. Effect of substrate of SN2 reactions. Factors affecting SN2 reactions: leaving group- Part 1. Factors affecting SN2 reactions: leaving group-Part 2. Factors affecting SN2 reactions: Leaving group- Part 3.

Source Image: slideplayer.com
Download Image
arrange the following compounds in order of sn2 reaction:1)ch3(ch2)3ch2Br; (2) (ch3)2chch2ch2Br ;3) ch3ch2ch(ch3)ch2Br;4) ch3(ch3)2cch2B Which compound in each of the following pairs will react faster in S N 2 reaction with ¯ O H? C H 3 B r o r C H 3 I (C H 3) 3 C C l o r C H 3 C l. Which compound in each of the following pairs will react faster in S N 2 reaction with ¯ O H?
Source Image: byjus.com
Download Image
Predict the compound in each pair that will undergo the SN2 react… | Channels for Pearson+
arrange the following compounds in order of sn2 reaction:1)ch3(ch2)3ch2Br; (2) (ch3)2chch2ch2Br ;3) ch3ch2ch(ch3)ch2Br;4) ch3(ch3)2cch2B Chemistry Chemistry questions and answers Which Sn2 reaction is each pair is faster? А. Оосно Br О. + Br Br Qocня. Br В. ci с сн,соо + Өон СІ ОН + This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Question: Which Sn2 reaction is each pair is faster? А.
Which would undergo SN2 reaction faster in the above pair and Why? Chapter 8: Nucleophilic Substitution – ppt download Jan 11, 20241) The reaction below follows the S N 2 mechanism. a) Write the rate law for this reaction. b) Determine the value of the rate coefficient, k, if the initial concentrations are 0.01 M CH 3 Cl, 0.01 M NaOH, and the initial reaction rate is 6 x 10 -10 M/s. c) Calculate the initial reaction rate if the initial reactant concentrations are changed