So we’re going to need 0.833 moles of molecular oxygen. And then I just multiply that times the molar mass of molecular oxygen. So, times 32.00 grams per mole of molecular oxygen. 0.833 times 32 is equal to that. If you go three significant figures, it’s 26.7. 26.7 grams of oxygen, of molecular oxygen.
Laundry Scoop 😉 : r/bathandbodyworks
Compute the product (s) for the following reaction. Predict the product for reaction: Ba (OH)_2+HNO_3 to. Predict the product of this reaction: Predict the product of the given reaction on dec-5-yne. Predict the product of this reaction below. Predict the product of the reaction: Cr_2O_3 (s) + OH^- (aq) + H_2O (l) to.
Source Image: chegg.com
Download Image
Solution. Step 1: Write a balanced equation after determining the products and reactants. In this situation, since we assume copper does not react, the reactants are only H + (aq) and Fe (s). The given product is H2 (g) and based on knowledge of redox reactions, the other product must be Fe 2+ (aq).

Source Image: youtube.com
Download Image
Predict the product of the following reaction. OH H+ In a chemical reaction, the reactant that is consumed first and limits how much product can be formed is called the limiting reactant (or limiting reagent). In this video, we’ll determine the limiting reactant for a given reaction and use this information to calculate the theoretical yield of product. Created by Sal Khan.

Source Image: slideshare.net
Download Image
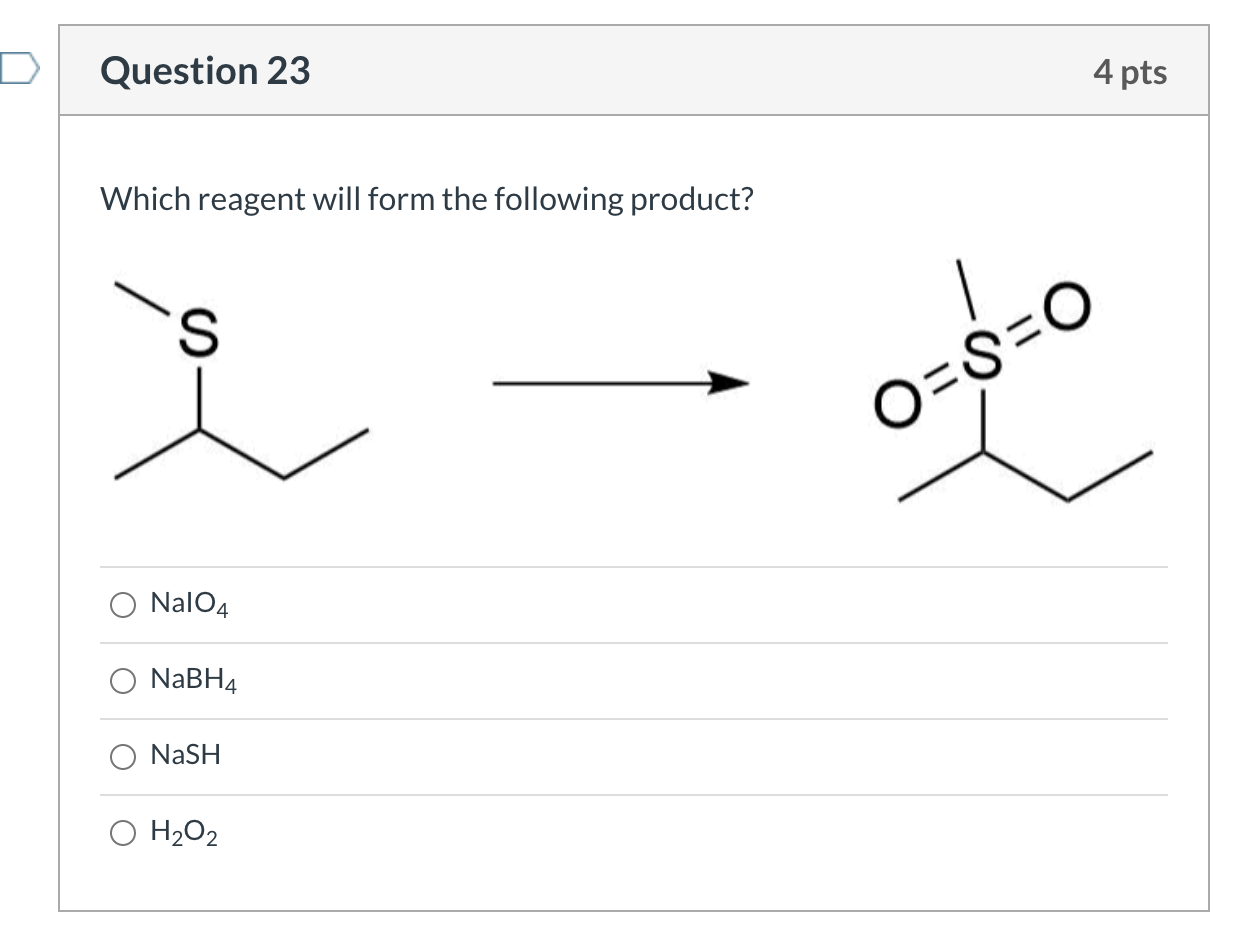
Determine The Product Of The Following Reaction
In a chemical reaction, the reactant that is consumed first and limits how much product can be formed is called the limiting reactant (or limiting reagent). In this video, we’ll determine the limiting reactant for a given reaction and use this information to calculate the theoretical yield of product. Created by Sal Khan. Determine the major product of the following reaction sequence: Determine the major products of the following reaction. Predict the product of the following reaction: 1) CH3MgBr, Et2O 2) Etl; Predict the product of the following reaction: 1) O3, CH2Cl2 2) H2O; Predict the product of the following reaction as seen below:
Kinetics | PPT
Question: 33. Determine the product of the following reaction. (1) O3 (2) Zn/H20 algehelea & ceny D. ОН H 32. What is (are) the product (s) of the reaction below? RCO3H H2O, HO HO HO HO A. HO B. HO HO- C. D. E A and D 31. Determine the product of the following reaction. МСРВА OH A. B. 용 C. D. OH ОН 30. Polymeric Material Selection: A Critical Factor In Making Successful Plastics Parts

Source Image: linkedin.com
Download Image
Organic Synthesis International: Atom Economy (AE), Reaction Mass Efficiency (RME) and Mass Intensity (MI) Question: 33. Determine the product of the following reaction. (1) O3 (2) Zn/H20 algehelea & ceny D. ОН H 32. What is (are) the product (s) of the reaction below? RCO3H H2O, HO HO HO HO A. HO B. HO HO- C. D. E A and D 31. Determine the product of the following reaction. МСРВА OH A. B. 용 C. D. OH ОН 30.
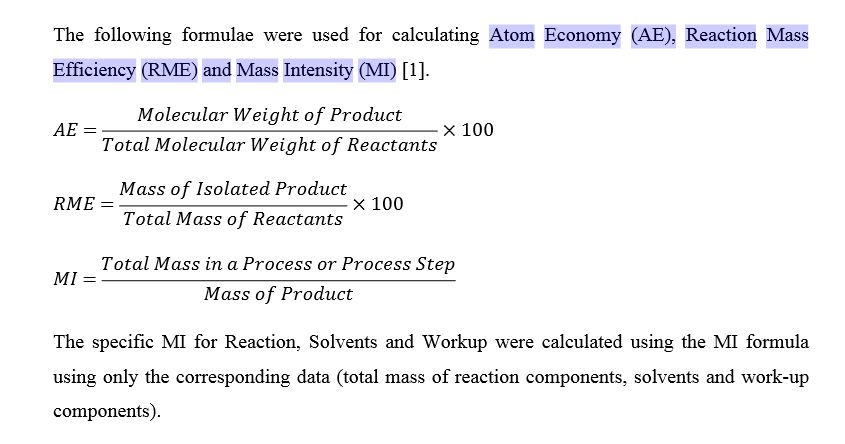
Source Image: organicsynthesisinternational.blogspot.com
Download Image
Laundry Scoop 😉 : r/bathandbodyworks So we’re going to need 0.833 moles of molecular oxygen. And then I just multiply that times the molar mass of molecular oxygen. So, times 32.00 grams per mole of molecular oxygen. 0.833 times 32 is equal to that. If you go three significant figures, it’s 26.7. 26.7 grams of oxygen, of molecular oxygen.

Source Image: reddit.com
Download Image
Predict the product of the following reaction. OH H+ Solution. Step 1: Write a balanced equation after determining the products and reactants. In this situation, since we assume copper does not react, the reactants are only H + (aq) and Fe (s). The given product is H2 (g) and based on knowledge of redox reactions, the other product must be Fe 2+ (aq).

Source Image: toppr.com
Download Image
Chemistry!!! Not Mystery : Alkene: Carbocation Rearrangement in Electrophilic addition Reaction If you li … View the full answer Previous question Next question Transcribed image text: Determine the product (s) of the following reaction. H2 Pd-C HII O land III I or II II and III O I, II, and III What is the reagent required to accomplish the following transformation?

Source Image: chemistrynotmystery.com
Download Image
Pharma Engineering: [How To] Select a Motor Capacity for Agitator In a chemical reaction, the reactant that is consumed first and limits how much product can be formed is called the limiting reactant (or limiting reagent). In this video, we’ll determine the limiting reactant for a given reaction and use this information to calculate the theoretical yield of product. Created by Sal Khan.
![Pharma Engineering: [How To] Select a Motor Capacity for Agitator](https://2.bp.blogspot.com/-VBzmFl1-APc/VybaznNzn3I/AAAAAAAAAlQ/iweZL4HYFvUJ_hWp3Nug6bKPxbO4eO_nwCLcB/s1600/Mud%2BAgitator.jpg)
Source Image: pharmacalculations.com
Download Image
Predict the product of the following reaction. OH H+ Determine the major product of the following reaction sequence: Determine the major products of the following reaction. Predict the product of the following reaction: 1) CH3MgBr, Et2O 2) Etl; Predict the product of the following reaction: 1) O3, CH2Cl2 2) H2O; Predict the product of the following reaction as seen below:

Source Image: toppr.com
Download Image
Organic Synthesis International: Atom Economy (AE), Reaction Mass Efficiency (RME) and Mass Intensity (MI)
Predict the product of the following reaction. OH H+ Compute the product (s) for the following reaction. Predict the product for reaction: Ba (OH)_2+HNO_3 to. Predict the product of this reaction: Predict the product of the given reaction on dec-5-yne. Predict the product of this reaction below. Predict the product of the reaction: Cr_2O_3 (s) + OH^- (aq) + H_2O (l) to.
Predict the product of the following reaction. OH H+ Pharma Engineering: [How To] Select a Motor Capacity for Agitator If you li … View the full answer Previous question Next question Transcribed image text: Determine the product (s) of the following reaction. H2 Pd-C HII O land III I or II II and III O I, II, and III What is the reagent required to accomplish the following transformation?